Case Study – NIHR Support
A little while ago, the NIHR supported Forte Medical Ltd on a usability study for the Peezy™ Mid-Stream Urine device – an easier, cleaner and… Read More

A little while ago, the NIHR supported Forte Medical Ltd on a usability study for the Peezy™ Mid-Stream Urine device – an easier, cleaner and… Read More

The latest issue of Urology News features an interesting and important conversation with Urological Consultant Mr Ased Ali about the importance of urine in diagnosis… Read More

Amongst British adults, urology diseases such as testicular cancer and prostate cancer are not well understood and men are not looking out for the signs… Read More

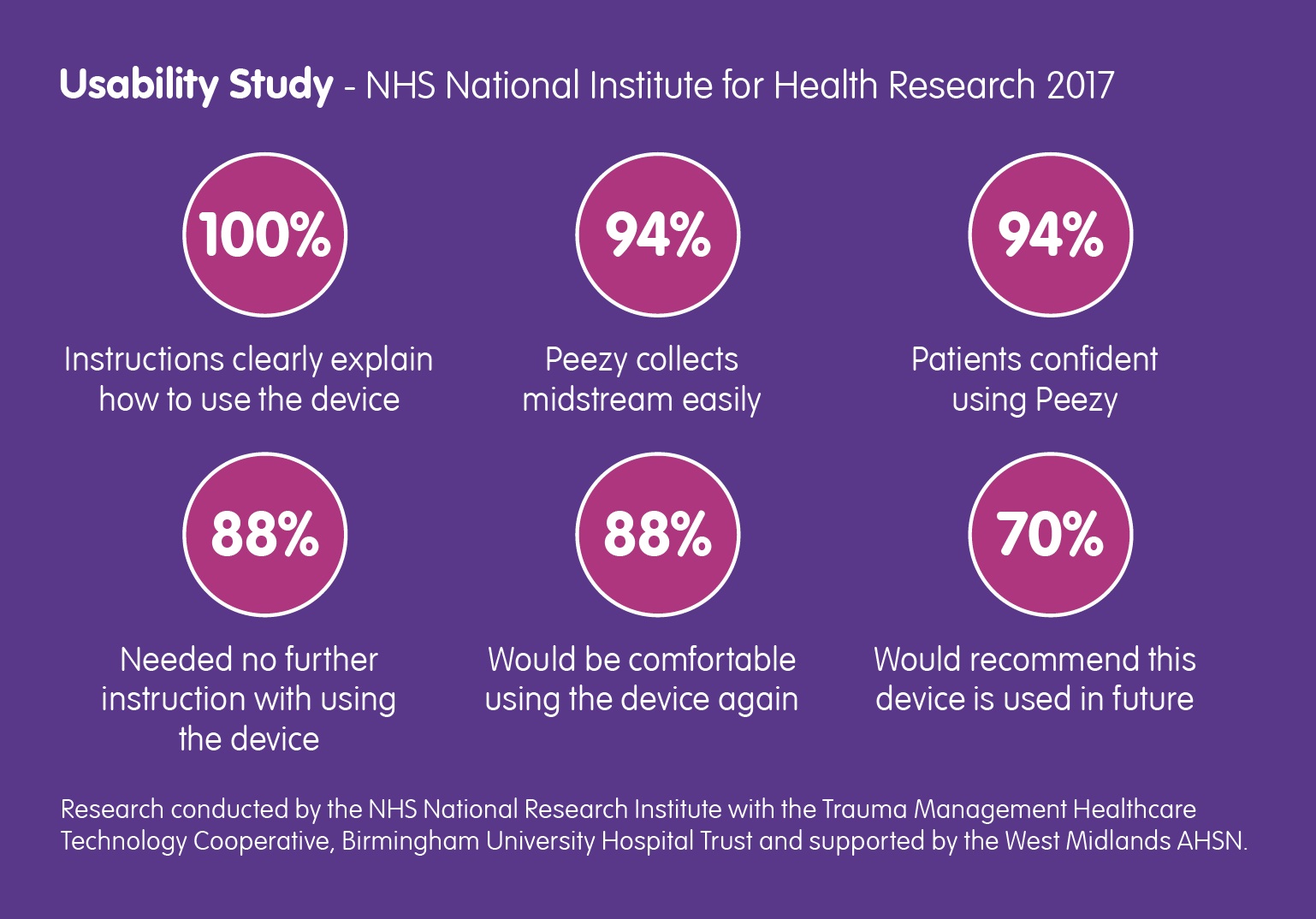
Back in March of this year, the NHS National Institute for Health Research contacted Forte Medical, asking for a case study… Read More
Interesting research being undertaken by Barbara Guinn, Head of Biomedical Sciences, University of Hull in The Conversation.com. “A urine test for ovarian cancer could… Read More

This is the latest recommendation following publication of a Californian study about the rise of antibiotic-resistant bacteria. But right-first-time diagnosis and treatment is only… Read More
A new study shows that Antibiotic-resistant UTI bacteria is becoming more common; Knowlex has created a short-film about the research and what you should know… Read More

We’ve been an advocate for the MUST Campaign since they launched in May and this article explains why it is so important. Read about it… Read More

The inaugural speech delivered by Mr Matt Hancock upon his appointment as Secretary of State for Health, focused quite rightly on the… Read More
Barry Shrier, Founder of annual health innovation event Giant Live, presents the third Disruptor Giant Health Innovators TV interview featuring Giovanna Forte, CEO of Forte… Read More